|
This page will (hopefully) contain answer keys to worksheets
if you have questions please e-mail me... type the question please my worksheets are at school!!


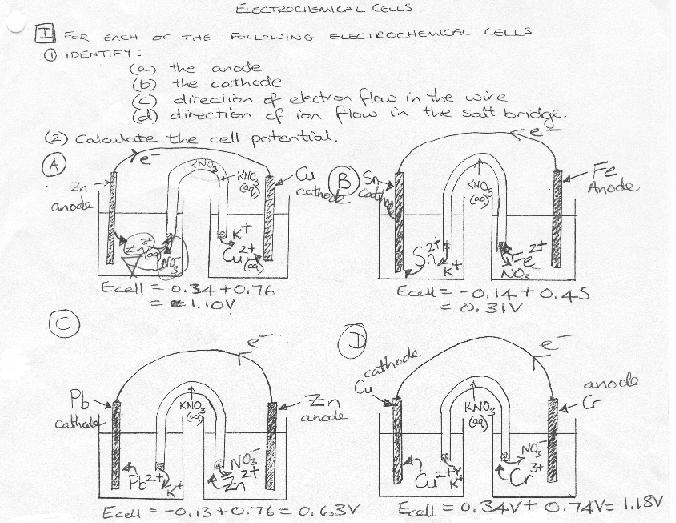
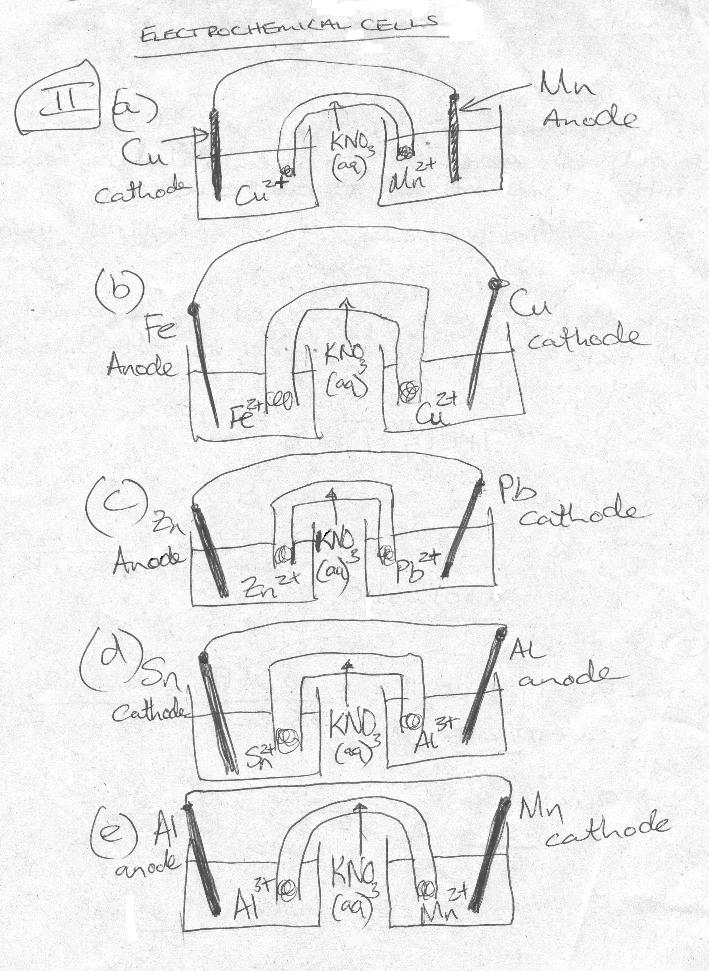
Note: for 2c and 2e - the reactions are ONLY spontaneous in acidic solution!


Acid Base Review KEY


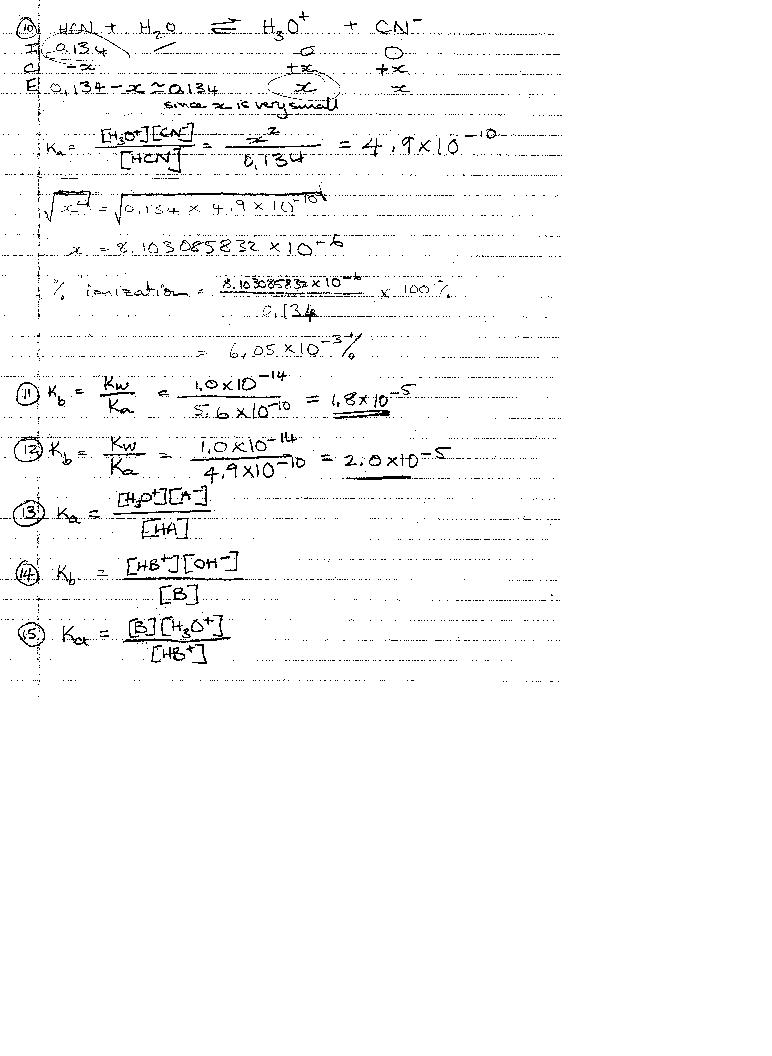

Correction to #17: the square root sign got missed out!
x = SQRT 2.6e-6
pOH = 2.8328
pH = 11.17


Chemistry 12 Equilibrium Review – Part I and II
Part I
1) No macroscopic changes, forward and reverse rxns still occur
2) Dynamics => forward and reverse rxns are still occurring => NOT static
3) solid and liquid
4)
Catalyst increase rate of rxn, establishes equm faster, has no effect on position of equm
5) Endothermic rxn consumes heat and Δ H is +
exothermic rxn produces heat and Δ H is -
6) Two
factors that control equilibria are ENTHALPY and ENTROPY... four possible combinations of these are: 1) Enthalpy pushes forward
& Entropy pushes forward => rxn goes to completion, 2) Enthalpy pushes forward & entropy pushes back => equm,
3) Enthalpy pushes back and entropy pushes forward => equm, 4) Enthalpy pushes back and entropy pushes back=> no rxn
7) Enthalpy pushes forward if rxn is EXOTHERMIC, enthalpy pushes
back if rxn is ENDOTHERMIC, entropy pushes forward if rxn has largest # of most random phase on products side, entropy pushes
back if rxn has largest # of most random phase on reactants side.
8) see your notes!!!!
9) see your notes & Hebden page
56!!!!
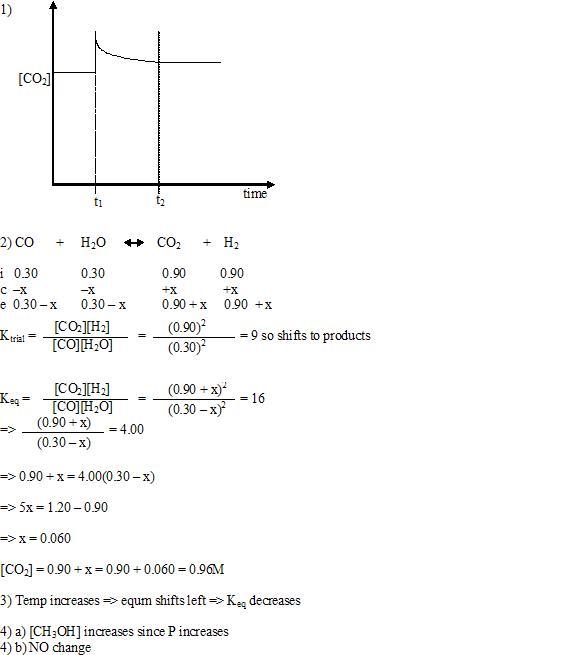
10) only temperature affects the value of Keq
11) Temp change:
endothermic
temp incr => shift right => Keq incr,
endothermic temp
decr => shift left => Keq decr
exothermic temp incr =>
shift left => Keq decr
exothermic temp decr => shift
right => Keq incr
>Stoichiometric ratios - depends on
rxn - ignore
>equation written in reverse => reciprocal
since reactants and products switch places
>catalyst - no
change
12) LARGE Keq => more products, less reactants, favours
products, enthalpy more likely to be pushing forward...
SMALL
Keq => less products, more reactants, favours reactants, enthalpy more likely to pushing backwards...
PART II - these are NOT questions - this is a LIST of quantitative skills you
should have!
CHEM
12 TRUE/FALSE questions
1. F 2. T 3. F 4. T 5. F 6. F 7. T 8. T 9. F 10. F 11. T 12. T 13. F Note for calculations: the
sig figs may be incorrect so if that is all that is wrong with your answer don't panic!


|

