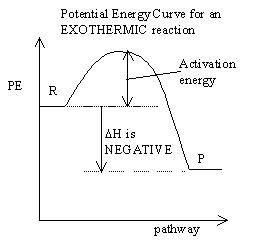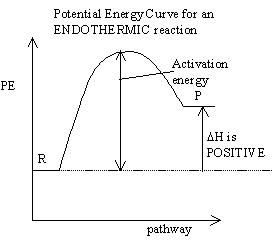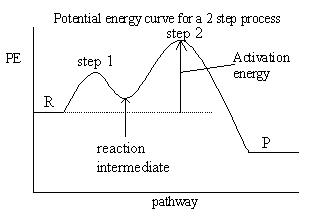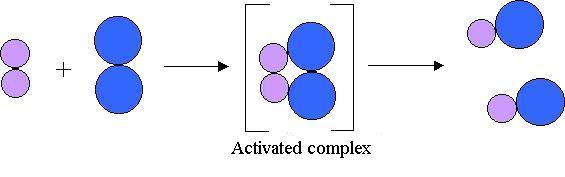
- factors affecting collision include:
- the energy of the collision: not all collisions are successful because energy is required to overcome the repulsion of the electron clouds surrounding the particles
- as temperature increases the kinetic energy of the particles increases. The particles move faster which causes collisions to occur more frequently and have more energy
- the orientation of the particles: atoms and molecules have reactive sites where reaction can occur, these active sites must be aligned or the collision will not be successful
- the concentration of the particles: the more particles there are the more collisions there will be which increases the probability of having successful collisions