- What is Dynamic Equilibrium?
- Why does equilibrium occur?
- Is it an
equilibrium or not?
- Shifting an equilibrium - Le Chatelier's
Principle
- Keq
- Keq meets Le Chatelier
- Calculator
stuff
Everything we talk about in dynamic equilibrium is directly connected
to what
you learned in the KINETICS unit. It is particularly important
that you
know how to ready PE graphs like the one below, and identify whether
the
reaction is exothermic or endothermic.
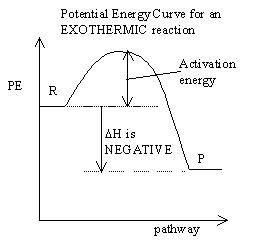
- Dynamic equilibrium is a reaction which
occurs both in the Forward direction AND in the Reverse direction
- the reaction is DYNAMIC because it
continues to occur even though there are no observable macroscopic
changes that can be observed
- the following properties will remain
constant when equilibrium has been established:
- temperature
- pressure
- concentration of reactants and products
- colour
- volume
- the rate of the forward reaction = rate of
reverse reaction at equilibrium
- equilibrium reactions do not go to
completion because the reverse reaction uses up products
You already know
that equilibrium
occurs because both the forward and the reverse reactions occur at the
same
time, and at the same rate. BUT why do both reactions occur at
all?
In the KINETICS unit, you learned:
- EXOTHERMIC reactions are SPONTANEOUS and go
to completion
- ENDOTHERMIC reactions are NOT spontaneous
and require a lot of energy in order to occur
So,
why does the
endothermic reaction in an equilibrium occur at all?
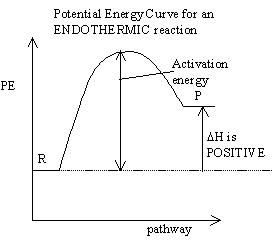
There are two forces that drive an equilibrium:
- ENTHALPY - the amount of energy released or
consumed during a reaction
- ENTROPY - the amount of randomness in a
system
Randomness is determined by the state the reactant or product is
in.
Gases are more random than solutions. Solutions are more random
than
liquids. Liquids are more random than solids.
Randomness summarized from most to least random:
Gas >> Aqueous >>
Liquid
>> amorphous solids > crystaline solids
Reactions ALWAYS tend to MINIMUM enthalpy and MAXIMUM entropy. If
these
two drives oppose each other then the reaction will be a dynamic
equilibrium.
If these two forces push in the same direction, the reaction will
either go to
completion, or not occur.
MINIMUM ENTHALPY: ΔH is NEGATIVE, or the heat
term is on the
PRODUCTS side of the equation.
MAXIMUM ENTROPY: ΔS is POSITIVE, or the products side is most
random.
(top)
In order to decide if a reaction is a dynamic equilibrium, you will
have to
determine the direction that ENTHALPY pushes and the direction that
ENTROPY
pushes. When these two driving forces oppose each other then
there is a
dynamic equilibrium. When enthalpy and entropy both push in the
forward
direction, the reaction will go to completion. When enthalpy and
entropy
both push in the reverse direction, the reaction will not occur.
In chemistry 12 you will be expected to determine whether a given
reaction is
likely to be an equilibrium or not. You will not have to
determine
anything about the position of the equilibrium or anything tricky, just
what
direction each driving force pushes.
Some examples:
A(l) + 2B(g) → AB2(g) enthalpy pushes → and entropy pushes ← so reaction is an equilibrium
A(g) + B(g) + heat → AB(g) enthalpy pushes ← and entropy pushes ← so there is NO reaction
AB(s) + heat → A(l) + B(g) enthalpy pushes ← and entropy pushes → so reaction is and equilibrium
A2B(g) → 2A(g) + B(g) + heat enthalpy pushes → and entropy pushes → so the reaction goes to completion
Shifting
an equilibrium - Le Chatelier's
Principle
Le
Chatelier's
principle states: An equilibrium system when subjected to
a
stress will
shift to counteract the stress and a new equilibrium will be
established.
So what does this
really mean? Well there are several stresses
that a
reaction can experience, the most common are changes in temperature,
pressure,
volume or concentration. Pressure and volume changes only affect
equilibria which include gas molecules. Temperature changes will
affect
any equilibrium system.
Some analogies to
help you understand what is going on:
- When you are too hot, your instinct is to
do something to cool down, equilibrium reactions have the same
instinct. An equilibrium reaction will always be exothermic in
one direction and endothermic in the opposite direction. Since
the endothermic direction "consumes heat" a reaction that has its
temperature raised will start to shift so that the endothermic
direction is favoured. The reaction shifts AWAY from the heat
term.
- When you are cold, you want to get warmer,
equilibrium reactions do the same thing. An equilibrium reaction
that is cooled down will always try to heat itself up. Since the
exothermic direction "produces heat" a reaction that is cooled down
will start to shift so that the exothermic direction is favoured.
The reaction shifts TOWARDS the heat term.
- When you are under a lot of pressure... ok
so are you starting to see a trend? yes? no? Let's
see if you get this... Will you try to relieve pressure or add
more? Equilbrium reactions will always try to relieve any build
up of pressure by shifting to the side of the reaction with fewer gas
molecules. Fewer gas molecules take up less space and so the
pressure will decrease.
What
you need to
do with a Le Chatelier's principle question:
- Identify the stress
- Decide how the stress will affect the
equilibrium
- Determine what direction the equilibrium
will have to shift in order to reduce the stress on the system
- Determine whether rates for the forward and
reverse reactions will increase, decrease, or stay the same
Some
examples:
1) N2O4
+ heat ↔ 2NO2
- As temperature rises the equilibrium shifts
AWAY from the heat term => more NO2 is produced.
The NO2 looks BROWN whereas the N2O4
is colourless.
- As temperature goes down the equilibrium
shifts TOWARDS the heat term => more N2O4 is
produced => colour fades
- As pressure increases the equilibrium
shifts to the side with FEWER gas molecules because this will relieve
the pressure => this equilibrium will shift to the reactants side
=> colour fades
- As pressure decreases the equilibrium
shifts to the side with MORE gas molecules because this will increase
the pressure => this equilibrium will shift to the products side
=> colour gets darker
- If [N2O4] is
increased there will be too much reactant so the equilibrium shifts
AWAY from the reactant side and towards the products side
- If [NO2] is decreased there will
be too little product so the equilibrium shifts TOWARDS the products
side.
2) Haber Process (see page 56 of Hebden)
3) Making CaO from limestone (see page 56 of Hebden)
The GRAPHS...
You
need to be able to understand the "concentration vs time graphs" that
show how concentration changes as a result of a stress. This
information is on pages
50 to 53 of Hebden, and in your class
notes. Make sure you can do question 27 on page 55 of
Hebden.
Example
Temperature was increased [A] was
increased
Pressure was increased
Summary of the graphs:
- all changes are gradual = TEMPERATURE change
- one concentration is a sudden increase or decrease = CONCENTRATION change
- all sudden increase
or sudden decrease = PRESSURE change
The
Equilibrium Constant - Keq
The
equilibrium
constant Keq is the ratio of the . A Keq
that is LARGER
than 1 means that the equilibrium favours products
over
reactants. A Keq that is
SMALLER than 1
means that the equilibrium
favours reactants over
products.
Writing a Keq
expression for an equation - only use species that are either gases or
aqueous, all other species do NOT appear in Keq expression.
CaCO3(s) ↔ CaO(s) + CO2
Examples:
- The value of Keq is ONLYaffected by
TEMPERATURE!
- We assume that Keq is constant when the concentration or gas pressure changes!
Keq is ratio of so if you have
conditions that result from an equilibrium being shifted, then you can
predict from the Ktrial what direction the
reaction needs to shift in order to reach equilibrium. You need
to either be given the Keq value or be given the
conditions at equilibrium before the shift occurs.
- If Ktrial is LARGER than Keq then there is too much product and not enough reactant ⇒ shifts to reactants
- If Ktrial is SMALLER than Keq then there is not enough product and too much reactant ⇒ shifts to products
- If Ktrial is equal to Keq then you are already at equilibrium ⇒ does NOT shift
Examples:
1) Calculating Keq
A 3.0L bulb contains 4.0mol gas A, 6.0mol gas B, and 2.3mol gas A2B
What is Keq for the equilibrium: 2A(g) + B ↔ A2B(g)
2) Calculating
Keq from initial and some
equilibrium values
5.0mol of gas A2B3 is introduced into a 3.0L
bulb. The following equilibrium is established: A2B3(g) ↔ 2A(g) + 3B(g)
At
equilibrium there are 1.5mol gas A. Determine Keq.
NOTE: Be careful with significant
figures sometimes the + − rule will affect the significant figures when
using ICE chart.
3) Calculating
initial concentrations using Keq
a) Gas AB2 was introduced into a 1.5L bulb. The following equilibrium was attained: AB2(g) ↔ A(g) + 2B(g)
At equilibrium [A] = 0.15M. If Keq = 0.89, how many moles of AB2 was originally present?
b)
Consider the equilibrium: 2A2B(g) ↔ 2A2(g) + B2(g)
A2B is placed in a 3.0L flask. At equilibrium [B2]
= 0.87M
How many moles of A2B were present initially if Keq
= 1.5
4) Determine
whether a system is at equilibrium using Ktrial and direction of shift
to establish equilibrium
Keq = 0.78 for the equilibrium
A2(g) + B2(g) ↔ 2AB(g)
If 1.5mol A2, 1.5mol B2 and 7.0mol AB are
put in a 3.0L flask which direction will the reaction shift to reach
equilibrium?
5) Calculating
Equilibrium concentration of species
Consider the equilibrium: AB(g) + CD(g) ↔ AD(g) + CB(g)
Keq = 2.8
If 3.5mol AB and 3.5mol CD are placed in a 2.0L bulb and the system is
allowed to come to equilibrium, what is the equilibrium concentration
for all species?
6) Finding New Equilibrium [ ] when
an existing equilibrium is shifted
a) Consider the equilibrium: A2(g) + B2(g) ↔ 2AB(g)
At equilibrium there are 3.0moles A2, 4.5moles B2
and 5.2moles AB in a 2.0L flask. 1.5moles A2 is added and
the system is allowed to re-establish equilibrium. Calculate the
concentration of all species when the new equilibrium is established.
b) Consider the equilibrium: 2A2B(aq) ↔ 2A2(g) + B2(aq)
At equilibrium there are 3.00moles A2B, 2.00moles A2
and 1.00moles B2 in a 1.0L flask. How many moles of A2
need to be removed to increase [B2] to 1.5M
For
more examples and explanations
please refer to HEBDEN pages 63 - 72.