(top)
Balancing half-reactions
The steps you should follow are:
- Balance MAJOR species using coefficients
- Balance O by adding H2O
- Balance H by adding H+
- Balance charge by adding e− to the side of the equation that has too much + charge
Example
1) Ru2+ → RuSTEP 1: major species balanced
STEP 2: no O in equation
STEP 3: no H in equation
STEP 4: add 2e− to LEFT side
| Ru2+ | + | 2e− | → | Ru |
2) Al2O3 → 2Al
| Al2O3 | + | 6H+ | + | 6e− | → | 2Al | + | 3H2O |
(top)
Balancing REDOX Equations − Half-Reaction Method
Steps:- Identify and write each half-reaction
- Balance each half-reaction
- Multiply each half-reaction equation so the number of electrons is equal
- Cancel electrons and other species that appear on both sides
Examples
1) Mn2+ + IO3− → MnO4− + I2 (acidic)| Mn2+ | + | 4H2O | → | MnO4− | + | 8H+ | + | 5e− |
| 2IO3− | + | 12H+ | + | 10e− | → | I2 | + | 6H2O |
_________________________________________________________
| 2×( | Mn2+ | + | 4H2O | → | MnO4− | + | 8H+ | + | 5e− | ) |
| 2IO3− | + | 12H+ | + | 10e− | → | I2 | + | 6H2O |
_________________________________________________________
| 2Mn2+ | + | 2 | → | 2MnO4− | + | 4 | + |
| 2IO3− | + | + | → | I2 | + |
_________________________________________________________
| 2Mn2+ | + | 2IO3− | + | 2H2O | → | 2MnO4− | + | 4H+ | + | I2 |
2) C2O42− + IO3− → CO2 + I2 (acidic)
| C2O42− | → | 2CO2 | + | 2e− |
| 2IO3− | + | 12H+ | + | 10e− | → | I2 | + | 6H2O |
_________________________________________________________
| 5×( | C2O42− | → | 2CO2 | + | 2e− | ) |
| 2IO3− | + | 12H+ | + | 10e− | → | I2 | + | 6H2O |
_________________________________________________________
| 5C2O42− | → | 10CO2 | + |
| 2IO3− | + | 12H+ | + | → | I2 | + | 6H2O |
_________________________________________________________
| 2IO3− | + | 5C2O42− | + | 12H+ | → | I2 | + | 10CO2 | + | 6H2O |
3) Balancing in basic solution: O2 + Cr(OH)3 → HO2− + CrO42− (basic)
| O2 | + | H+ | + | 2e− | → | HO2− |
| Cr(OH)3− | + | H2O | → | CrO42− | + | 5H+ | + | 3e− |
_________________________________________________________
| 3×( | O2 | + | H+ | + | 2e− | → | HO2− | ) |
| 2×( | Cr(OH)3− | + | H2O | → | CrO42− | + | 5H+ | + | 3e− | ) |
_________________________________________________________
| 3O2 | + | 3H+ | + | → | 3HO2− |
| 2Cr(OH)3− | + | 2H2O | → | 2CrO42− | + | 10H+ | + |
_________________________________________________________
| 3O2 | + | 2Cr(OH)3− | + | + | 2H2O | → | 3HO2− | + | 2CrO42− | + | 7 |
_________________________________________________________
| 3O2 | + | 2Cr(OH)3− | + | → | 3HO2− | + | 2CrO42− | + | ||
| + | 7OH− | + | ||||||||
| ‾‾‾‾‾‾‾‾ | ||||||||||
| 5 |
_________________________________________________________
| 3O2 | + | 2Cr(OH)3− | + | 7OH− | → | 3HO2− | + | 2CrO42− | + | 5H2O |
(top)
Determining Oxidation Numbers
Finding the oxidation number of a substance is like finding the "charge" or combining capacity of an atom in a molecule.Rules
- Elements ALWAYS have oxidation number ZERO
- Hydrogen is ALWAYS +1
- Alkali ions are ALWAYS +1
- Alkaline Earth ions are ALWAYS +2
- Oxygen is USUALLY −2, but is −1 in peroxides
- ALL OTHERS MUST BE CALCULATED! Use the sum of charges of species
Example
HPO42−H = +1 P = x O = −2
⇒ 1 + x + 4×(−2) = −2
⇒ x = +5
(top)
Balancing REDOX Equations − Oxidation Number Method
See Hebden!



(top)
REDOX Titrations
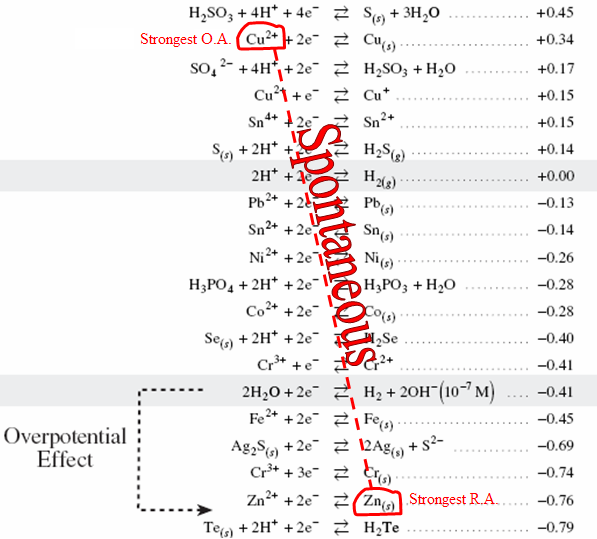
Uses stoichiometry of REDOX reactions to find the concentration of an unknown species.Most Common Oxidizing Agents
MnO4− and Cr2O72− because they both change colour when they reduce ⇒ these substances act as an indicator as well as a reactant.Example
A 25.00mL sample of an aqueous Fe2+ salt solution is titrated using acidified KMnO4. 24.83mL of 0.7864M KMnO4 was required to react all of the Fe2+. What is the [Fe2+]?| 5×( | Fe2+ | → | Fe3+ | + | e− | ) |
| MnO4− | + | 8H+ | + | 5e− | → | Mn2+ | + | 4H2O |
_________________________________________________________
| 5Fe2+ | + | MnO4− | + | 8H+ | → | 5Fe3+ | + | Mn2+ | + | 4H2O |
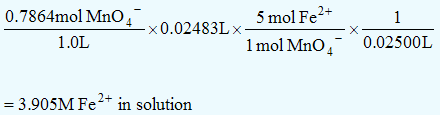
Most Common Reducing Agent
I− is used as a reducing agent then the I2 can be titrated since starch will indicate for the presence of I2. This takes place in two reactions:REACTION 1: Producing I2 Using Excess I− (note: substance that is our unknown is the LIMITING REAGENT!)
| 2I− | → | I2 | + | 2e− |
| H2O2 | + | 2H+ | + | 2e− | → | 2H2O |
_________________________________________________________
| 2I− | + | H2O2 | + | 2H+ | → | I2 | + | 2H2O |
REACTION 2: Titrating the I2
| I2 | + | 2e− | → | 2I− |
| 2S2O32− | → | S4O62− | + | 2e− |
_________________________________________________________
| 2S2O32− | + | I2 | → | S4O62− | + | 2I− |
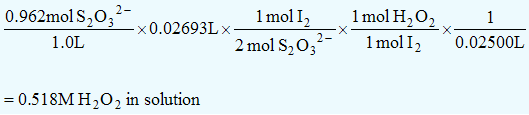
(top)
Electrochemical Cells
Electrochemical cells are SPONTANEOUS redox reactions, they produce a flow of electrons. This is what happens when you use a battery in a cellphone or camera.The parts of an electrochemical cell are:
- Beaker with one half reaction that will be the reduction reaction.
- Beaker with one half reaction that will be the oxidation reaction.
- Voltmeter and wires to allow electron flow
- Salt Bridge containing KNO3 or NaNO3 to allow ion flow
Steps:
- Determine which half-reaction is the oxidation and which one is the reduction.
- The OXIDATION reaction occurs at the ANODE. The REDUCTION reaction occurs at the CATHODE.
- Label the anode and cathode.
- Label the direction of ion flow
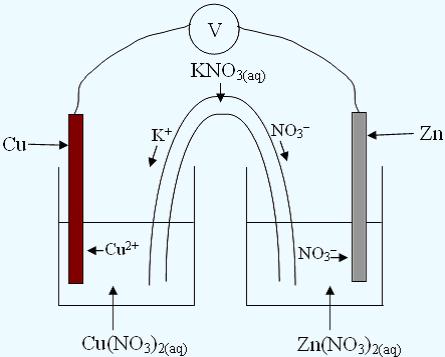
In the diagram electrons flow FROM the anode TO the cathode through the wire.
Cations flow TOWARDS the cathode. Anions flow TOWARDS the anode.
Memory Aid
An Ox chases a Red Cat
Cell Potential
- Since we have a POTENTIAL DIFFERENCE there needs to be a point of comparison. The standard chosen is the HYDROGEN half-reaction. This is assigned a value of ZERO, and all other values are determined based on this standard.
- Use table of reduction potentials to determine the potential of a cell.
- Calculate the cell potential using: Eocell = Eored − Eoox
- Cell potential is POSITIVE for a SPONTANEOUS REDOX reaction.
The cell potential for the diagram is calculated using: Eocell = Eored − Eoox = +0.34 − (−0.76) = 1.10V
Corrosion
Corrosion is a spontaneous redox reaction which causes a metal to oxidize. When iron corrodes this process is called rustingThe corrosion of iron is shown in the diagram below:

You should note that corrosion is really an application of an electrochemical cell that forms in the presence of oxygen and moisture.
Corrosion Prevention
- Using a barrier ⇒ prevents moisture and oxygen from reaching the metal by adding a coating
- paint
- plastic coatings
- grease
- Cathodic Protection = Sacrificial Protection = Anodizing
- attach a more active metal which will corrode preferentially
- used for pipelines and large structures like oil rigs as well as household objects
- galvanizing is a specific example of cathodic protection where iron is coated with Zn.
- Change the chemical environment
- remove moisture
- remove oxygen
- increase the pH
(top)
Electrolysis
In an electrolysis the reaction is NOT spontaneous ⇒ electricity is required to make the reaction occur.There are THREE different types of electrolytic cell that you will be expected to know about
- Electrolysis of a Molten (Melted=Liquid) Binary Salt
- Electrolysis of an Aqueous Solution with INERT electrodes (Graphite or Pt)
- Electrolysis of an Aqueous Solution with REACTIVE electrodes
Electrolysis of a Molten Salt
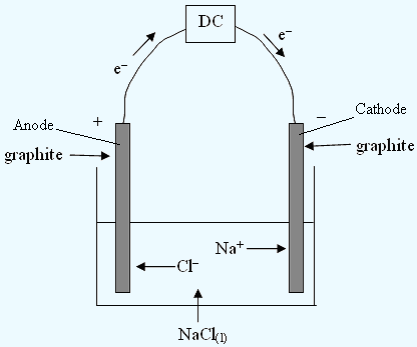
| ANODE reaction: | 2Cl− | → | Cl2 | + | 2e− | Cathode reaction: | Na+ | + | e− | → | Na |
Electrolysis of an Aqueous Salt With Inert Electrodes
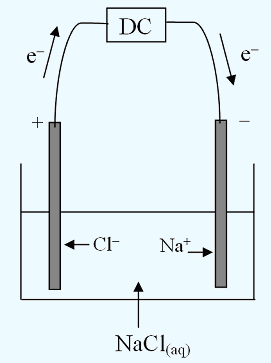
| ANODE reaction: | 2Cl− | → | Cl2 | + | 2e− | Cathode reaction: | Na+ | + | e− | → | Na | ||||
| OR: | |||||||||||||||
| H2O | → | ½O2 | + | 2H+ | + | 2e− | 2H2O | + | 2e− | → | H2 | + | 2OH− |
Look at the table and find the CLOSEST together REDOX pair. In this case the closest pair are:
| ANODE reaction: | 2Cl− | → | Cl2 | + | 2e− | Cathode reaction: | 2H2O | + | 2e− | → | H2 | + | 2OH− |
Electrolysis of an Aqueous Salt With Reactive Electrodes
1) Electroplating:
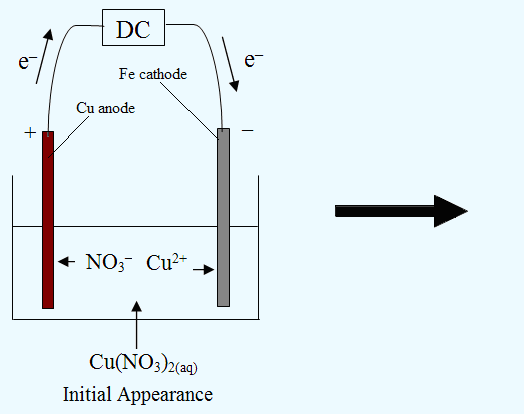
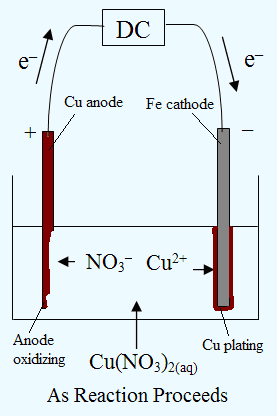
2) Electrorefining
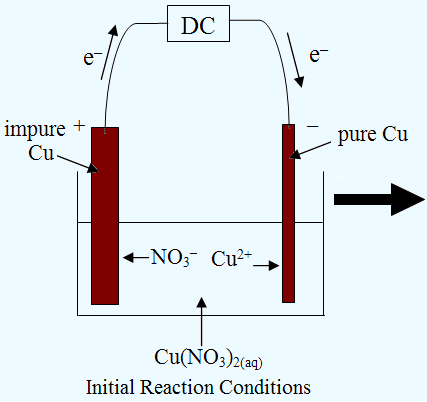
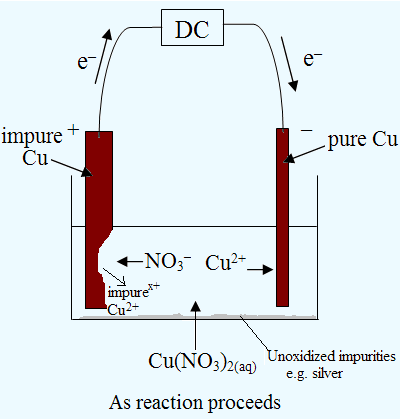
(top)