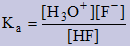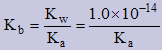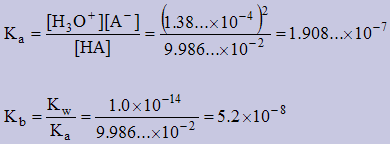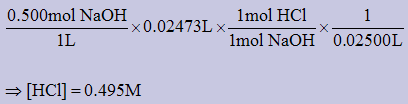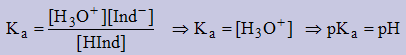Acid-Base Chemistry
You have probably heard the words acid and base used in an everyday context, and most of you could say that vinegar is an acid. However, what do these terms mean?
In this unit we will look at:
Arrhenius Acids and Bases
General Properties of Acids and Bases
The Nature of H+
The Meaning of Strong and Weak
Bronsted-Lowry Acids and Bases
Conjugate Pairs of weak acids and weak bases
Amphiprotic Species
Qualitative Uses of Relative Strength Table
Equilibrium Constant for Ionization of Water
pH and pOH
Mixtures of strong acids and bases
Equilibrium Constants for weak acids, Ka and weak bases, Kb
Calculations involving Ka and Kb
Hydrolysis of salts
Titrations and calculations
Titration Curves
Indicators
Buffers
pH of Metal and Non-Metal Oxides
Acid Rain
Arrhenius Acids and Bases
The definitions for Arrhenius Acids and Bases are the ones you should already have come across in Junior Science and possibly chemistry 11. These definitions are:
Acid: − any substance that releases H+ in water.
Base: − any substance that releases OH− in water.
Salt: − any ionic substance that is not an acid or base.
When an acid reacts with a base you get the following general reaction.
ACID + BASE → SALT + WATER
Examples:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
2H3PO4(aq) + 3Ca(OH)2(aq) → Ca3(PO4)2(aq) + 6H2O(l)
(top)
Properties of Acids and Bases
General Properties
| Properties of Acids | Properties of Bases |
| react with base | react with acids |
| turn litmus RED | turn litmus BLUE |
react with some metals
- main ones are Mg, Al, Zn, Fe, Sn, Pb | react with Al and Zn only |
| corrosive - will damage skin | feel slippery/soapy - they are turning you into soap! |
| electrolyte ⇒ conduct electricity | electrolyte ⇒ conduct electricity |
| taste sour | taste bitter |
Specific Properties
You are expected to know some specific properties for common acids and bases. This information is on pages 112 − 114 of Hebden. This is good chemical knowledge to have if you are planning to study chemistry beyond grade 12.
Often college/university teachers will assume that you know this information!
(top)
The Nature of H+
H+ is able to stick to H2O to form what is called a hydronium ion, H3O+
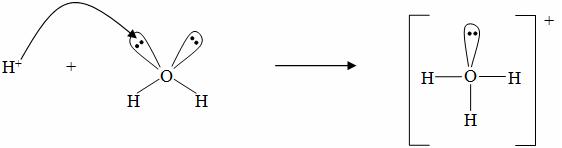
The purpose of doing this is to allow H2O to be written into the equation:
HCl(aq) + H2O(l) → H3O+(aq) + Cl−(aq)
(top)
Strong & Weak Acids and Bases
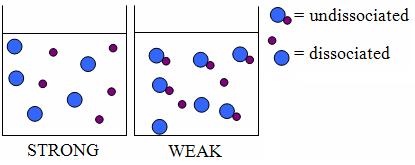 In chemistry the word STRONG has a specific meaning that is distinct from regular English. This is important for you to remember.
In chemistry the word STRONG has a specific meaning that is distinct from regular English. This is important for you to remember.
STRONG refers to an acid or base that dissociates (splits into ions) completely in aqueous solution.
WEAK refers to an acid or base that does NOT dissociate completely in aqueous solution.
It is very important that you do NOT use the words strong and weak to refer to concentration. If you want to describe the relative concentration of a solution use the word concentrated to describe a high concentration and dilute to describe a low concentration.
(top)
Bronsted-Lowry Acids and Bases
Since the weak acids and bases do not fit into the Arrhenius definition of an acid, the definition of acids and bases was expanded by Johannes Nicolaus Bronsted and Thomas Lowry.
The definitions that they created for acids and bases were:
Acid: proton donor
Base: proton acceptor
These definitions allowed weak acids such as vinegar (acetic acid) to be included in the definition of acids and bases
Examples:
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq)
|
| acid | | base | | acid | | base |
| NH3(aq) | + | H2O(l) | ↔ | NH4+(aq) | + | OH−(aq)
|
| base | | acid | | acid | | base |
Describing the donation of protons
Acids can be described as being:
| Monoprotic: | − acid can donate 1 proton |
| Diprotic: | − acid can donate 2 protons |
| Triprotic: | − acid can donate 3 protons |
| Polyprotic: | − acid can donate > 1 proton |
(top)
Conjugate Pairs of Weak Acids and Weak Bases
When a weak acid dissociates the weak base that is produced is called the conjugate base of the acid.
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq)
|
| weak acid | | | | | | conjugate base |
When a weak base dissociates the weak acid that is produced is called the conjugate acid of the base.
| NH3(aq) | + | H2O(l) | ↔ | NH4+(aq) | + | OH−(aq)
|
| weak base | | | | conjugate acid | | |
(top)
Amphiprotic Species
A species which is able to behave as BOTH an acid and a base is described as amphiprotic. If we look at the behaviour of water in the two equations below:
Equation 1:
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq)
|
| acid | | base | | acid | | base |
Equation 2:
| NH3(aq) | + | H2O(l) | ↔ | NH4+(aq) | + | OH−(aq)
|
| base | | acid | | acid | | base |
In the first equation water is behaving as a base, and in the second equation water is behaving as an acid.
Amphiprotic species are: water and any negative ion that contains an H, except for HSO4−. The reason HSO4− is NOT amphiprotic is because HSO4− cannot act as a base because it comes from a STRONG acid ∴ HSO4− is not part of an equilibrium as a base and will only behave as an acid.
(top)
Qualitative Uses of Relative Strength Table
One of the things I will expect you to do is use the table to determine if an equilibrium involving a weak acid and weak base with their conjugates will favour products or reactants. Since the equilibria will always shift away from the strongest acid this is easy to determine.
Most of the work that we will do with the Table of Relative Acid Strengths will be quantitative − that is using numbers and doing calculations − BUT you can use the table to get information without having to number crunch.
Examples:
1) Mixing HF and NH3
| HF(aq) | + | NH3(aq) | ↔ | NH4+(aq) | + | F−(aq)
|
| stronger acid | | | | weaker acid | | |
So this equilibrium favours the PRODUCTS
2) Mixing HSO3− with F−
| HSO3−(aq) | + | F−(aq) | ↔ | SO32−(aq) | + | HF(aq)
|
weaker
acid | | | | | | stronger acid |
So this equilibrium favours the REACTANTS
(top)
Equilibrium Constant for Ionization of Water
Water ionizes as follows:
| 2H2O−(l) | ↔ | H3O+(aq) | + | OH−(aq)
|
Since this is an equilibrium, we can write an equilibrium expression, and since the expression is for water we refer to the expression as Kw
Kw = [H3O+][OH−] = 1.0×10−14 at 25∧C
| Neutral solution | [H3O+] = [OH−] |
| Acidic solution | [H3O+] > [OH−] |
| Basic solution | [H3O+] < [OH−] |
(top)
pH and pOH
The numbers that are used for concentration of hydrogen ion or hydroxide ion can be very small, so use the pH and pOH scale to describe how acidic or basic a substance is.
pH is based on logs, so here is a quick summary:
| log10 1.0×102 = 2 |
| log10 1.0×10−9 = −9 |
Each whole number on a logarithmic scale is the exponent of a power of ten ⇒ if the value of the log changes by 1 the original number has changed by a multiple of ten.
| pH = −log[H3O+] |
| [H3O+] = antilog (−pH) |
| pOH = −log[OH−] |
| [OH−] = antilog (−pOH) |
pH + pOH= pKw
⇒ pH + pOH= 14 |
Any time you see a small p in front of something in this unit, it means take the −log of the something...
To reverse taking the negative log ⇒ you will use antilog on your calculator. This is done by pressing:
| SHIFT log and then the number | OR... | the number then SHIFT log | OR... | using the 10x button |
Significant Figures
Since pH and pOH are calculated using logs, the WHOLE number basically tells you the value of the exponent and the DECIMAL PLACES tell you the precision of the number ⇒ you ONLY count the decimal places of the pH or pOH to determine sig figs!
Calculations involving pH and pOH:
1) The pH of a solution is 1.98. Find the [H3O+]
[H3O+] = antilog (−pH) = antilog (−1.98) = 1.0471285480508995334645020315281×10−2 = 1.0×10−2M
2) What is the concentration of OH− in a base solution that has a pH of 13.45?
pH = 3.45 ⇒ pOH = 14 − 3.45 = 10.55
∴ [OH−] = 2.8183829312644538191019236991551×10−11 = 2.8×10−11M
3) Find the pH of 0.00543M solution of a STRONG monoprotic acid.
[H3O+] = 0.00543M
pH = −log[H3O+] = −log 0.00543 = 2.2652001704111530524152284203326
∴ the pH of the solution is 2.265 (to 3 sf)
4) Find the pH of a 0.0129M solution of NaOH
[OH−] = 0.0129M ⇒ pOH = −log(0.0129) = 1.8894102897007510362998839435157
pH = 14 − 1.8894102897007510362998839435157 = 12.110589710299248963700116056484
∴ the pH of the solution is 2.111 (to 3 sf)
(top)
Mixtures of Strong Acids and Bases
When a strong acid and a strong base are mixed, you are only concerned with which one is in excess. The amount of the excess will tell you enough information to calculate the pH of the resulting mixture.
Example
15.0mL of 1.35M HCl is mixed with 50.0mL of 0.89M NaOH. What is the pH of the mixture?

(top)
Equilibrium Constants for Weak Acids, Ka,
and Weak Bases, Kb
Since weak acids and weak bases form an equilibrium with their conjugate a Keq can be written. Since this is a special case for weak acids and weak bases we use Ka for the weak acids and Kb for the weak bases.
Acids:
→ H3O+ equation is same as table just add H2O to reactants and change H+ to H3O+
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq)
|
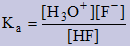
Bases:
→ OH− equation has to be re-written by reading table from right to left and adding H2O to left and OH− to right.
| CN&minus(aq) | + | H2O(l) | ↔ | HCN(aq) | + | OH−(aq)
|

Note: Ka×Kb = Kw ⇒ We can use this information when calculating the value of Kb since these are not given on the data table.
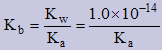
(top)
Calculations involving Ka and Kb
Example 1
Determine the pH of a 0.73M solution of HF.
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq) |
| I | 0.73 | | --- | | 0 | | 0 |
| C | −x | | | | +x | | +x |
| E | 0.73−x | | | | x | | x |
| ≐ 0.73 |
| since x is v. small |

Example 2
% Ionization
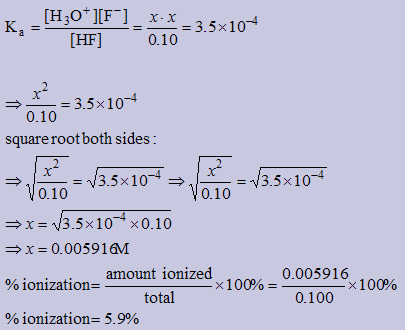
Calculate the % ionization of a 0.10M HF solution
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq) |
| I | 0.10 | | --- | | 0 | | 0 |
| C | −x | | | | +x | | +x |
| E | 0.10−x | | | | x | | x |
| ≐ 0.10 |
| since x is v. small |
Example 3
The pH of a weak base B− is 8.13 for a 0.100M solution. What is the Kb for the base? (assume the base is monobasic)
pOH = 14.00 − 8.13 = 5.87 ⇒ [OH−] = antilog (−5.87) = 1.34896...×10−6
| B&minus(aq) | + | H2O(l) | ↔ | HB(aq) | + | OH−(aq) |
| I | 0.100 | | --- | | 0 | | 0 |
| C | −1.34...×10−6 | | | | +1.34...×10−6 | | 1.34...×10−6 |
| E | 9.999...×10−2 | | | | 1.34...×10−6 | | 1.34...×10−6 |

Example 4
The pH of a 0.100M solution of a weak monoprotic acid, HA, is 3.86. Calculate the Kb of the conjugate base?
pH = 3.86 ⇒ [H3+] = antilog (−3.86) = 1.38038426...×10−4
| HA(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | A−(aq) |
| I | 0.100 | | --- | | 0 | | 0 |
| C | −1.38...×10−4 | | | | +1.38...×10−4 | | 1.38...×10−4 |
| E | 9.986...×10−2 | | | | 1.38...×10−4 | | 1.38...×10−4 |
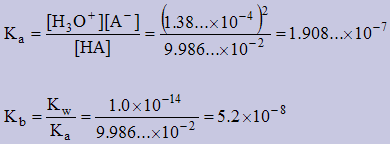
(top)
Hydrolysis of Salts
A salt has two ions − a cation (+) and an anion (−). Sometimes these ions will interact with water when dissolved in aqueous solution.
Cations
The cations which hydrolyse can be found on the table of Relative Acid Strengths NO OTHER POSITIVE IONS WILL HYDROLYSE except for the four ions on this data table, these + ions are: Fe3+, Cr3+, Al3+, and NH4+
Anions
Any negative ion that is the conjugate base of a weak acid will hydrolyse, again these are ion that are found on the table of relative acid strengths.
One thing to be careful about is that some negative ions are amphiprotic so you will need to be aware of this and compare the Ka and Kb values. Which ever is larger will dominate the solution so,
Ka > Kb = acidic
Ka < Kb = basic
Ka = Kb = neutral
Both ions Hydrolyse
If both ions hydrolyse then you will have to compare the Ka and Kb values
Ka > Kb = acidic
Ka < Kb = basic
Ka = Kb = neutral
Examples
1) NaHCO3
NaHCO3(aq) → Na+(aq) + HCO3−(aq)
- We know that Na+ does NOT hydrolyse because it is not on the table.
- We know that HCO3− is amphiprotic and will therefore behave as either an acid or a base ⇒ we will have to determine Ka and Kb
Ka = 5.6×10−11 and Kb = 2.3×10−8 ⇒ solution is basic
2) AlCl3
AlCl3 → Al3+ + 3Cl−
- We know that Al3+ behaves as an ACID
- We know that Cl− does NOT hydrolyse
∴ the solution is ACIDIC
3) NH4CH3COO(aq)
NH4CH3COO(aq) → NH4+(aq) + CH3COO(aq)
- We know that NH4+ hydrolyses ⇒ Acidic
- We know that CH3COO− hydrolyses ⇒ Basic
- Need to compare Ka and Kb
Ka = 5.6×10−10 and Kb = 5.6×10−10
∴ since the Ka and Kb values are equal, the solution is neutral!
(top)
Titrations and Calculations
When a titration is carried out, you use STOICHIOMETRY of the equation to find missing a concentration.
Examples
1) A 25.00mL sample of hydrochloric acid was titrated with 24.73mL of 0.500M sodium hydroxide. What is the concentration of the HCl solution?
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(aq)
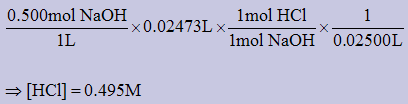
2) A 25.00mL sample of Sr(OH)2 was titrated with 18.95mL of 0.513M HCl. What is [Sr(OH)2]?
2HCl(aq) + Sr(OH)2(aq) → SrCl2(aq) + 2H2O(aq)

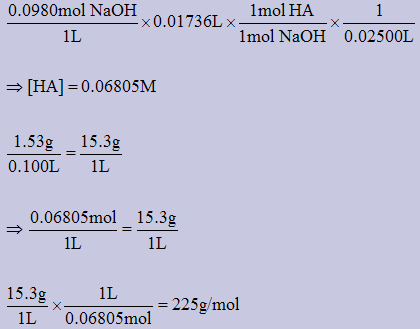
3)A 1.53g sample of a weak monprotic acid, HA, is dissolved to give a 100.0mL solution. A 25.00mL sample of this solution was titrated with 17.36mL of a 0.0980M NaOH solution. Determine the molar mass of the acid.
HA(aq) + NaOH(aq) → NaA(aq) + H2O(aq)
(top)
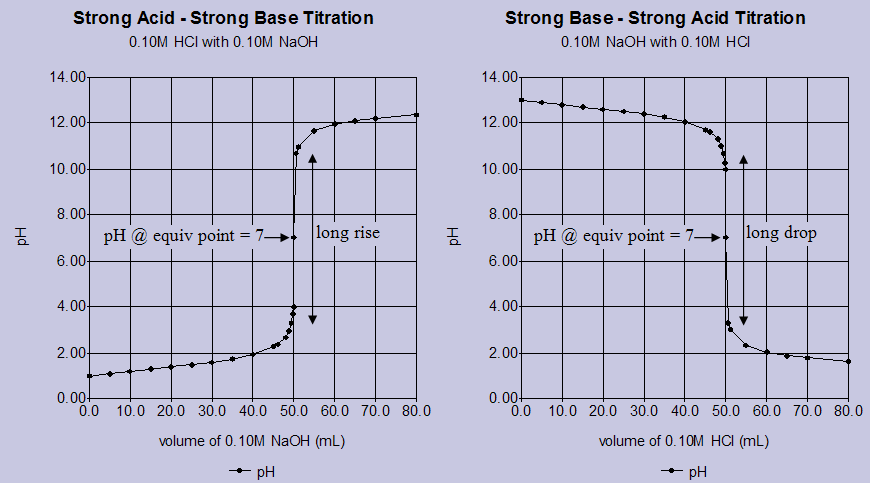
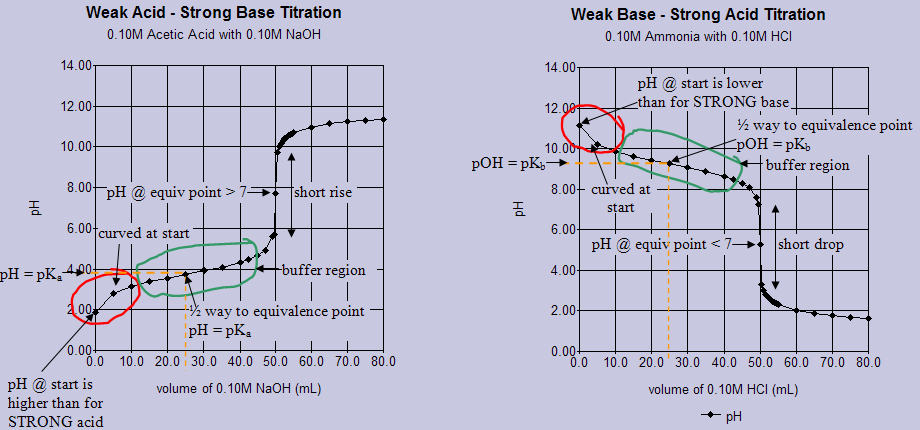
Titration Curves
(top)
Indicators
Indicators are weak organic acids or bases that have different colours for the acid and base forms.
| HInd(aq) | + | H2O(aq) | ↔ | H3O+(aq) | + | Ind−(aq)
|
| RED | | | | | | BLUE |
If acid is added to this system
[H3O+] increases ⇒ equilibrium shifts LEFT ∴ the [HInd] increases and the solution becomes RED
[HInd] > [Ind−]
If base is added to this system
[H3O+] decreases because the H3O+ reacts with the OH− ⇒ equilibrium shifts RIGHT ∴ the [Ind−] increases and the solution becomes BLUE
[HInd] < [Ind−]
Using Indicators to Find approx pH of a solution
1) A solution has the following effect on indicators
| Indicator | Colour |
| Methyl orange | Yellow |
| Bromcresol green | Blue |
| Bromthymol blue | Yellow |
Determine the approximate pH of the solution
| Indicator | Colour | pH |
| Methyl orange | Yellow | > 4.4 |
| Bromcresol green | Blue | > 5.4 |
| Bromthymol blue | Yellow | < 6.0 |
∴ the approximate pH of the solution is between 5.4 and 6.0
2) A solution of unknown pH is added to thymol blue which becomes green. Determine the pH of the solution.
Thymol blue is green in the middle of the range 8.0 − 9.6 ⇒ the pH of the solution is approximately 8.6
Finding the Ka of an indicator
In the middle of the pH range for the indicator [HInd] = [Ind−]
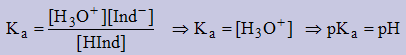
Example: Find the Ka of Neutral Red.
pH range = 6.8 − 8.0 ⇒ middle value = 7.4 ⇒ Ka = antilog (−7.4) = 3.981×10−8
∴ Ka of the indicator Neutral Red is approximately 4×10−8
(top)
Buffers
Buffer solutions are designed to have a KNOW pH that is resistant to change when small amounts of a contaminant are added.
Buffers are prepared by either mixing a weak acid or base with a salt of its conjugate OR by titrating the weak acid or base to halfway to the equivalence point.
Example of a buffer system would be HF mixed with NaF
This gives the equilibrium:
| HF(aq) | + | H2O(l) | ↔ | H3O+(aq) | + | F−(aq) |
When [HF] = [F−] the buffer has maximum capacity.
NOTE: it is important to remember that although the equilibrium equation used to explain how a buffer behaves is the same as a weak acid or base, the concentrations of the conjugate are different - we have deliberately increased the concentration of the conjugate.
What happens to our Buffer System when stressed
Add ACID − If acid is added to the system, the [H3O+] initially increases causing the equilibrium to shift LEFT, which causes the [H3O+] to decrease again ⇒ pH remains constant.
Add BASE − If base is added to the system, the [H3O+] initially decreases causing the equilibrium to shift RIGHT, which causes the [H3O+] to increase again ⇒ pH remains constant.
(top)
pH of Metal and Non-Metal Oxides
Metal Oxides | Non-Metal Oxides | Metalloids |
| basic | acidic | amphiprotic |
Possible exceptions would be Fe2O3, Al2O3, and Cr2O3 which are amphiprotic because the metal ion behaves as an acid.
(top)
Acid Rain
Causes
Acid Rain is caused by the presence of NON-METAL oxides that are not normally part of the atmosphere. These gases are NO, NO2, SO2, and SO3.
The pH of normal rainwater is about 5.4 due to dissolved CO2 which is naturally part of the atmosphere. If rain has a pH that is LESS than 5.4 then it is called acid rain.
Sources of Gases
There are a variety of sources for these gases including:
| Sources of NOx | Sources of SOx |
| spark plugs in cars | burning fossil fuels |
| lightning | volcanic eruptions |
Acids Formed
H2SO3, H2SO4, HNO2, and HNO3
You'll probably notice that two of these acids are STRONG acids ⇒ cause large drop in pH.
Environmental Impact
- Kills fish in lakes or rivers that do not have natural protection
- Damage to buildings, statues, etc.
- Damages plants and can even kill trees
Natural Protection
Areas with limestone deposits offer natural protection because the limestone will react and neutralize the acid.
Buffer systems can occur between dissolved CO2 (which forms H2CO3) and HCO3−
(top)
Acid Base Chemistry Tutorials

 In chemistry the word STRONG has a specific meaning that is distinct from regular English. This is important for you to remember.
In chemistry the word STRONG has a specific meaning that is distinct from regular English. This is important for you to remember.